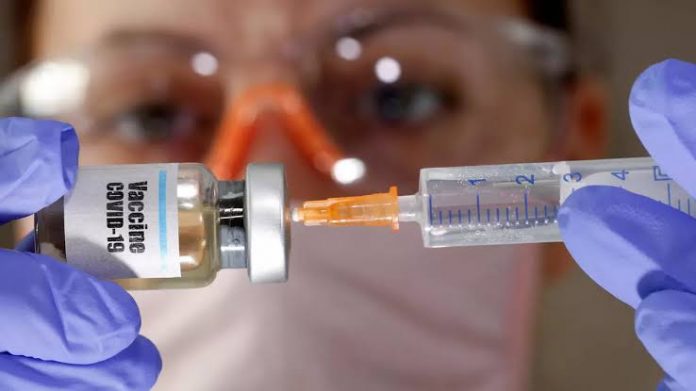
A leading pharmaceutical company, AstraZeneca has promised to develop an effective vaccine to a greater extent by the end of the year to battle the pandemic.
AstraZeneca’s chief executive Pascal Soriot stated vaccination to cure Coronavirus disease known as COVID-19 would be made later this year for limited use.
The leading medicine manufacturing company expressed concurrence to offer the vaccine being prepared by Oxford University as soon as it is approved effective.
Human trial of potential vaccination first began last week at Oxford University and many of the people had presented themselves voluntarily for the trial. Soriot stated, today’s prior concern is to get the vaccine produced to eliminate the virus.
University’s strategic team has a remarkable record so far and has a great potential in their advanced technology. Everything will be crystal-clear regarding its approach for vaccine development till the mid of June or July.
AstraZeneca has tied up with the university’s team and stats from testing could be seen in the mid of June, he added.
The conjoint agreement is to present the immunization for quick track administrative endorsement in the final quarter of 2020, and for it to be prepared for constrained use before the current year’s over.
The UK government has funded 200 million pounds in order to proceed with the vaccine preparation.
Science Experts say if the human trial of vaccines gets succeeded, it would be prepared on a large scale.
Furthermore, Professor of Medicine at Oxford University, Sir John Bell said, ” the human-trial facts and figures will be available as soon as mid-June.”
Censure is aimed to “see whether we can get this vaccine both to work and be manufactured and distributed to both the UK and globally,” he added.
Collaboration with AstraZeneca is to ensure the production of 10 million vaccines later this year, if the trial gets successful.
“Our production size is not that vast that we can build huge numbers of vaccines, this association with AstraZeneca will help endorse the manufacturing of doses at a high level,” he said.











[…] In the trial, Interleukin 7 will be given to severely ill patients with the largely low level of T l… as it has been tested and proved to be successful in safely increasing the level of defensive cells. […]
[…] intends to begin coronavirus vaccine clinical trials inside about fourteen days on immunization to battle the novel coronavirus, the Health Ministry said […]
[…] best suit their needs. This is an important breakthrough that could revolutionize the way we treat depression and other mental health conditions. So why is this study so important? Because currently, there is […]