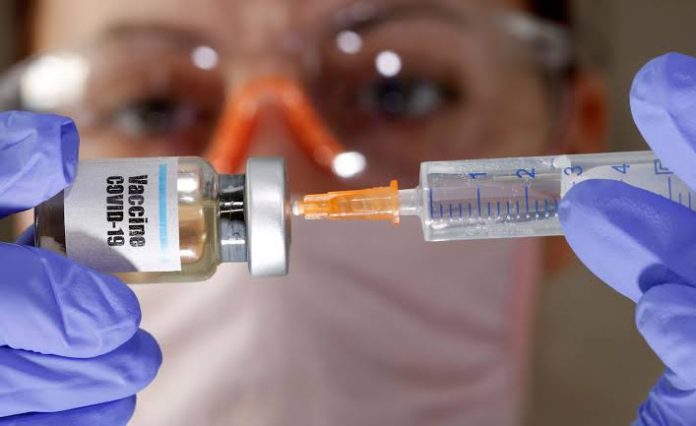
An Oxford vaccine for antibody against coronavirus is demonstrating effective results, findings of early-stage medical trials recommend.
The beginning-phase trial indicated the immunization is safe, causes a few negative symptoms, and prompts solid resistant reactions, results issued in The Lancet Journal.
But, researchers cautioned it is still too early to know whether the outcomes are sufficient, and major preliminaries are presently under the way.
The vaccine, named AZD1222 and being worked on by AstraZeneca and researchers at Oxford, didn’t immediate any genuine side effects and elicited neutralizer and T-cell immune reactions, as per preliminary outcomes.
It is produced using a hereditarily built virus that causes the regular cold in chimpanzees, which is then designed to express the coronavirus spike protein to trigger a solid invulnerable reaction in the human body.
The UK government has just ordered 100 million doses of the vaccine.
Oxford’s Covid-19 vaccine produces a good immune response, reveals new study.
Teams at @VaccineTrials and @OxfordVacGroup have found there were no safety concerns, and the vaccine stimulated strong immune responses: https://t.co/krqRzXMh7B pic.twitter.com/Svd3MhCXWZ— University of Oxford (@UniofOxford) July 20, 2020
Preliminaries including 1,077 sound grown-ups between April 23 and May 21 demonstrated the infusion prompted them to making antibodies and white defensive cells that can battle COVID-19. It didn’t create any genuine side effects
Prof Sarah Gilbert, from the University of Oxford, UK, stated: “There is still much work to be done before we can confirm if our vaccine will help manage the COVID-19 pandemic, but these early results hold promise.”
AstraZeneca’s is among the chief vaccine applicants against COVID-19, that has asserted in excess of 600,000 lives around the world, along with others in mid and late-phase preliminaries.
These incorporate shots being created by China’s Sinovac Biotech, another from state-claimed Chinese firm Sinopharm, and one from the US-based biotech firm Moderna.
AstraZeneca has consented with governments around the globe to deliver the vaccine should it demonstrate successful and garner administrative endorsement.
NEW—UK’s #COVID19 vaccine is safe and induces an immune reaction, according to preliminary results https://t.co/rDPlB9fDKr pic.twitter.com/z2t9Aubjim
— The Lancet (@TheLancet) July 20, 2020
The UK Government has just signed a consensus to assure the safety of about 100 million doses of the vaccines while the US has a contract agreement for 300 million of the early 1 billion doses developed.
UK’s Prime Minister Boris Johnson said the declaration was “very positive news”, yet in addition cautioned there are still further preliminaries to occur.
This is very positive news. A huge well done to our brilliant, world-leading scientists & researchers at @UniofOxford.
There are no guarantees, we’re not there yet & further trials will be necessary – but this is an important step in the right direction.https://t.co/PRUTu8rlPF— Boris Johnson #StayAlert (@BorisJohnson) July 20, 2020
The organization has said it won’t try to get profit from the vaccine during the pandemic.
Specialists said the vaccine caused minor reactions more much of the time than in a benchmark group — yet a dose of these could be decreased by taking paracetamol — with no genuine unfavorable impacts from the vaccine.
“Today’s data increases our confidence that the vaccine will work and allows us to continue our plans to manufacture the vaccine at scale for broad and equitable access around the world,” AstraZeneca’s Mene Pangalos said.











[…] old version of essential debris is regular with this statement. but, those theories of debris and their interactions spoil down while we try to use them for the bodily phenomena […]